
System Selection Guide
Mirion offers guidance with selections to meet your needs, based on application, software and hardware.
Mirion offers guidance with selections to meet your needs, based on application, software and hardware.
April 15, 2015 | By Mirion Technologies

Radioactive material is fairly common in nature, and generally pretty harmless in that state. It’s important to have an understanding of how much of the radioactive material a person comes across in a given day is from the natural environment around them.
One of the most commonly encountered sources of natural radiation is one that many people don’t suspect. Bananas, being naturally very high in potassium, consequently have a higher than usual amount of potassium-40, a radioactive isotope. In fact, the term “banana equivalent dose” has come into pretty common use as a reference point for communicating radiation exposure.

Other common foods that contain elevated levels of radioactive elements include carrots and white potatoes, which carry slightly lower levels of potassium-40. Lima beans have almost 50% more potassium-40 than bananas, and also very small amounts of radon-224. The food with the highest concentration of radioactive elements, in this case radium, is the Brazil nut. In all these foods, however, the levels are extremely low and not considered harmful, and almost none of the radioactive material consumed while eating any of them is retained in the body. The fat content of the Brazil nuts would pose a health risk long before the radiation in them, for example.
Powered by a continuous nuclear reaction, it comes as little surprise that the sun gives off quite a bit of radiation. There’s also a good bit of cosmic radiation origination from sources outside the solar system. Luckily for life on earth, however, most of that energy is intercepted and absorbed by the Earth’s magnetosphere and the ozone layer.
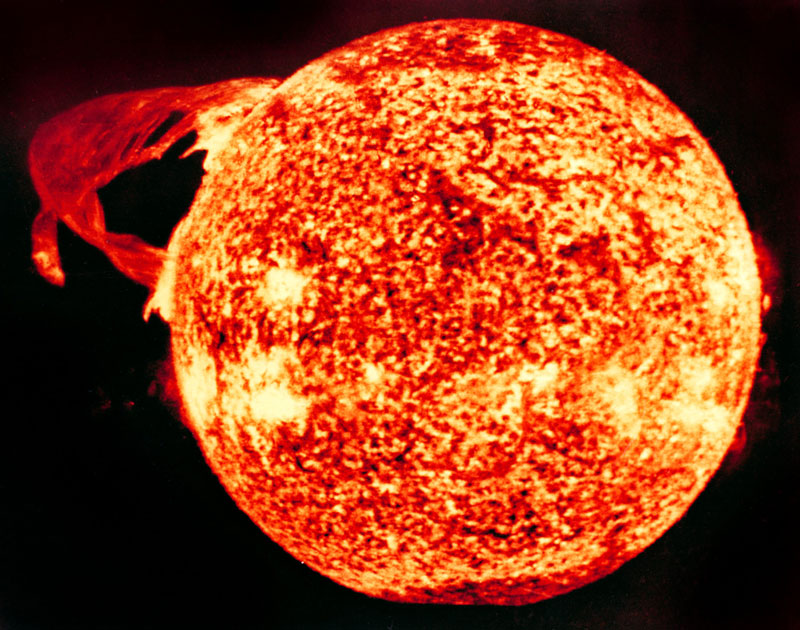
Cosmic radiation does, however, make up a small percentage (about 13%) of the total annual background radiation a person is exposed to over the course of a year. This exposure rate is slightly increased by living at higher altitudes, and even more so by air travel. Flight crews on long-distance, high-altitude flights tend to accumulate about 30% more annual radiation exposure than the average person.
(Radium, uranium, from Mining/Oil)
The other major source of naturally occurring radiation is from minerals and materials buried in the earth. Most common are potassium-40, uranium-238, and thorium-232, which all have fairly long half-lives. Additionally, there are small quantities of shorter-lived materials, such as radium-226, which is a decay product of U-238, and radon-222, which is a product of Ra-226. Radon, being a gas, can become a problem in some houses and other buildings, seeping in usually through cracks in solid foundations, and accumulating in rooms with poor ventilation. In the United States, the Appalachian Mountains region of southeast Pennsylvania, along with parts of Iowa, have the most problems with radon concentration, due to a larger deposit of uranium in the bedrock of those areas.

Another area where terrestrial radiation can become something of an issue is in the oil and natural gas mining industries, where it is referred to as Naturally Occurring Radioactive Material, or NORM. Often dredged up in pipes in process of mining exploration and production, radioactive elements like radon and radium will bind with molecules in fluid being used, bringing it up to the surface and potentially contaminating pipelines, tanks, and other equipment.
It may come as a surprise to some people, but since the human body is made up of many of the same atoms and elements that are found in the rest of the terrestrial environment, a certain percentage of those atoms are radioactive. Most common are Carbon-14, since life is carbon-based, and Potassium-40, since Potassium forms an important part of DNA molecules.

The presence of radioactive Carbon-14 in living organisms actually forms the basis of radiocarbon dating of organic material, due to the fact that levels of C-14 in plants and animals match ambient levels in the atmosphere at the time of their death. Afterwards, however, the C-14 decays (with a half life of around 5730 years) and by measuring the remaining amount, and comparing it with known or calculated amounts at different times. Through this method, the age of a sample up to around 45,000 years can be fairly accurately determined.
Looking for Services or Support?
We're here to help.